What is salen?
(don’t worry, this article starts off on a bit of a tangent on the background of salen complexes before we actually make some. this is still a synthesis writeup in the end and you will see these pretty coordination compounds just a few paragraphs away)

Salen is the contracted name of N,N′-bis(salicylidene)ethylenediamine, which is a compound made by condensation of salicylaldehyde and ethylenediamine (commonly shortened to en, don’t ask why). However, as of today, the original Salen ligand is much less relevant than the ligand class it shares its name with.
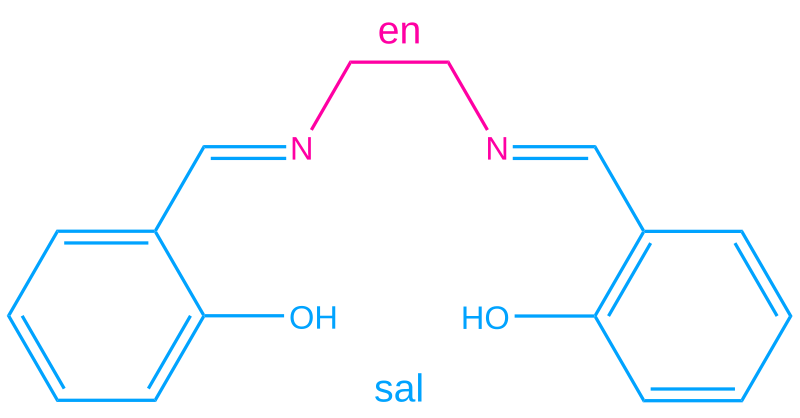
Really, nowadays you are more likely to encounter the terms “salen” or “salen-type” in the meaning that a compound either has the hydroxyaldehyde-diamine backbone or just the general vibes of a salen - a schiff base derived from some sort of carbonyl and a diamine, an example being the acacen subclass of salens derived from acetylacetone (abbreviated in coordination chemistry as acac) (though some like to treat them as a separate thing).
Salen ligands are just one small part of a large family of ligands, coexisting in it with a large amount of other catchily-named ligand types which differ in saturation (salan, salalen), the character of the diamine component (salophens, salpns), or even some that distinguish themself by being made from something other than a diamine (salomens). This list is far from exhaustive, and the terminology of salens isn’t standardized by anyone like IUPAC either way so godspeed if you try to dig deeper.

Why is salen?
Why bother? I ask myself that question far too much often to count. However unlike myself, salens have a quite wide range of uses1, both in and outside of research chemistry. In industry salen by itself is often used as a fuel additive to remove redox-active metal ions like copper out of solution. In chemistry, salen-type ligands are often used for catalysis, with the most prominent example being the chiral Jacobsen’s Catalyst used in the aptly named stereoselective Jacobsen Epoxidation. The preparation of the catalyst is simple enough to be part of the curriculum for undergraduate inorganic chemistry2, which leads us to the next point of why salens matter. And that being the fact that a lot of them are dead easy to make, making it a trivial task to derivatize them by varying substituents on the reagents and to tailor them to your needs (solubility, sterics, chirality etc), or to combinatorically make a giant library of them for whatever purposes people want giant chemical libraries (fancy linear algebra or some shit).
Another quite interesting salen complex is salcomine, derived from SalenH2 and a cobalt salt. Salcomine is (as far as I could gather)3 the first instance of a wholly synthetic complex that is able to bind dioxygen in a way somewhat similar to heme in hemoglobin. Though the coordination geometry differs, as unlike with heme, the dioxygen ligand bridges two molecules of salen instead of just being coordinated to one metal centre like in heme.
How is salen? (Let’s make some and find out!)
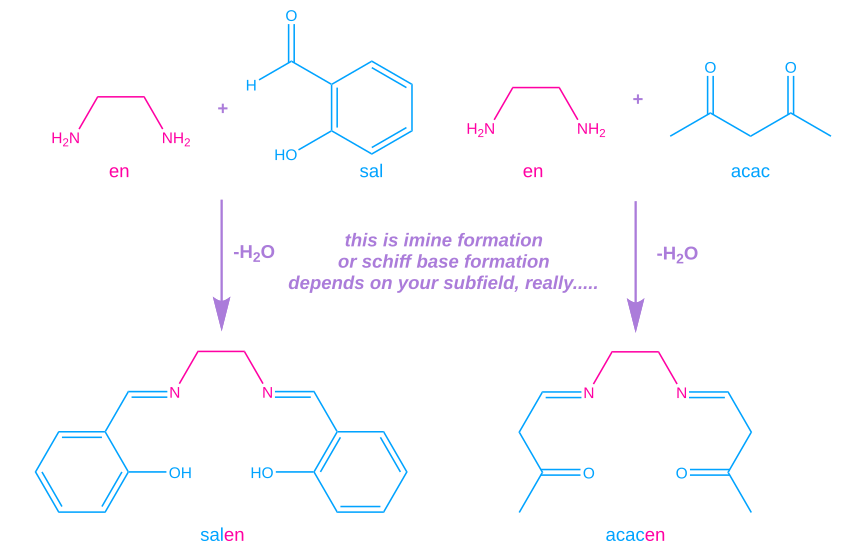
To follow up on the last few paragraphs, lets actually make some salens! Herein we describe the synthesis of 2 (arguably) salens, the original SalenH2 and AcacenH2, a schiff base derived from Acetylacetone and Ethylenediamine.
Salen
This procedure is taken from some random undergrad practical we found on the internet, which we could not track down again. The practical itself cites H. Diehl, C. C. Bach, G. C. Harrison, L. M. Limmett, Iowa State Coll. J. Sci. 1947, 21, 278, however we couldn’t track a digitized copy of that paper down on the internet either.
7.32 grams (60 mmol) of salicylaldehyde were dissolved in 60 ml of methanol and added to a solution of 1.8 grams (30 mmol) of ethylenediamine in 30 ml of methanol. The solution quickly becomes yellow and deposits a large amount of mildly fluorescent product of the same colour. The reaction mix is set in a fridge for a few hours and the product is later filtered off on a schott funnel.
After drying in air, the final yield of the salen ligand is 7.13 g (88%, 8.04g theoretical)

Acacen
This one is taken from a paper by Ozkar et al.4.
1.8 grams of neat ethylenediamine were added to 6 grams of Acetylacetone leading to a considerable evolution of heat and a formation of a yellow melt. On it’s cooling, yellow crystals were obtained, which were recrystallized from a small amount of hot distilled water. After filtering, a yield of 3.88 grams of an off-white product smelling strongly of bread was obtained (57%, 6.72 g theoretical).
The final ligand was a boring, sleep-inducing off-white powder, which however looked utterly stunning during crystallization

When is salen (complex formation)?
These are prepared in very similar ways from Copper (II) Acetate, with the workup differing slightly. Why acetates you may ask? In addition to acetate being a labile ligand, metal acetates provide a convenient pH buffer in reactions with acidic ligands, with the acetate ion acting as a base and shifting the equilibrium away from the salenH2 proligand towards a neutral [Cu(salen)] salt instead.
Acacen:
110 mg of Copper Acetate monohydrate were dissolved in methanol, to which a concentrated solution of 0.112 mg (0.5 mmol) of Acacen in the smallest possible amount of methanol was added. The color quickly changed to a deep purple and the solution was gently evaporated on a hotplate to near dryness. This product is strongly soluble in methanol and was crashed out by adding isopropanol and distilled water to the strongly concentrated solution in methanol (~2 ml). The resulting purple solid was filtered off on a schott funnel and dried on the pump and then in air. 90 mg of the complex were obtained, corresponding to a yield of 62% (143 mg theoretical)

Salen:
110 mg (0.55 mmol) of Copper Acetate Monohydrate was dissolved in ~5 ml of hot methanol and warm a solution of 134 mg of Salen (0.5 mmol) in 5 ml of methanol was added to it in one portion. The solution quickly darkened and a black-green-gold precipitate of Cu(salen) was observed. This was filtered off on an itsy-bitsy tiny wittle schott funnel, washed with methanol and dried in air. 120 mg of a stunning sparkly powder were obtained, corresponding to a yield of 72% (165 mg theoretical)

Conclusions, and what now?
Two ligands and two complexes of them were prepared. We can say so ourselves that they do look quite pretty, which really is the main goal of inorganic chemistry. Next up - probably the syntheses of the complexes of other metals with these exact same ligands (Nickel is apparently a quite nice red), which we didn’t perform because we couldn’t be bothered to make the appropriate acetates. And also, at some point, probably salcomine - either when we get around to setting up a proper lab bench with equipment for air-free chemistry or maybe following some sketchy old prep that claims to make it in your normal oxygenated atmosphere. Either way, we’re honestly just glad to get Acacen out the way, as a failed attempt at making this exact complex was performed last summer and it kept weighing on my psyche like a rather hefty axe above my head, waiting for the opportunity to finally sever our head from our torso.
Sources
-
https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/chemistry-and-synthesis/cross-coupling/salen-ligands ↩
-
knew a mate who did it at oxford in undergrad ↩
-
Tokuichi Tsumaki (1938). “Nebenvalenzringverbindungen. IV. Über einige innerkomplexe Kobaltsalze der Oxyaldimine”. Bulletin of the Chemical Society of Japan. 13 (2): 252–260. https://doi.org/10.1246/bcsj.13.252 ↩
-
Özkar, S., Ülkü, D., Yıldırım, L. T., Biricik, N. & Gümgüm, B. Crystal and molecular structure of bis(acetylacetone)ethylenediimine: intramolecular ionic hydrogen bonding in solid state. Journal of Molecular Structure 688, 207–211 (2004). https://doi.org/10.1016/j.molstruc.2003.10.016 ↩